An innovative process
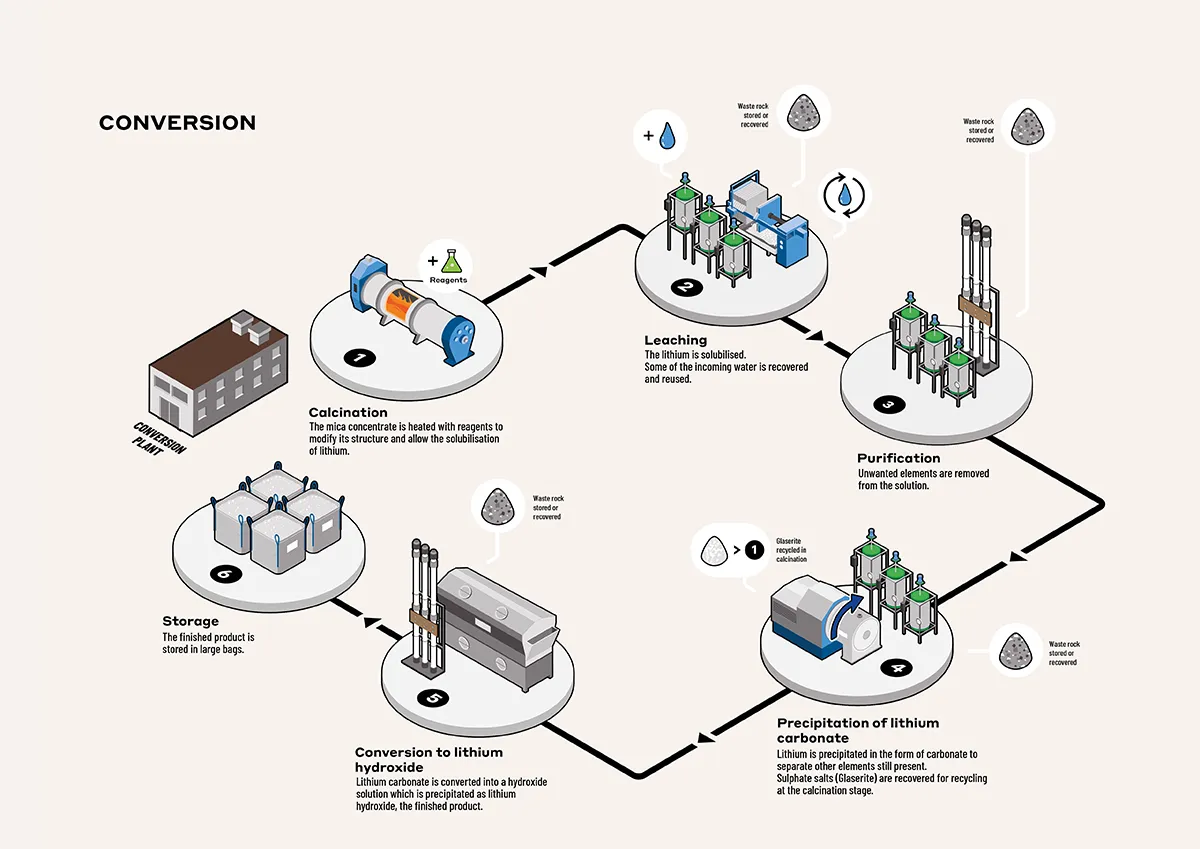
The conversion of lithiniferous mica concentrate into lithium hydroxide consists in separating the various elements found in the mica (iron, aluminium, silicates, potassium and lithium oxide). A number of steps are involved: a thermal process, calcination (which avoids the use of large quantities of acids), then a succession of treatments using water, which also limits the use of acid: leaching, purification and crystallisation.
The processes feature the technique of Zero Liquid Discharge into the environment, and utilisation of the residue from conversion (in particular for construction, snow removal, and in fertilisers).
Brownfield land
The La Loue site near Montluçon meets many criteria: sufficient surface area (nearly 40 ha) for the siting of industrial facilities, the railway terminal served by the national rail network, access to the motorway network, and connection to the electrical grid.
Its proximity to the concentration site is also a definite asset for revitalisation of the region.
Conversion in numbers
Production
Tonnes of lithium hydroxide produced annually
Employment
Employees on the conversion site
Dust
Percentage of dust reduction
Your questions about conversion
What measures will be taken to prevent industrial risks?
The teams will be trained in the processes, operation of the equipment, and risk control procedures. They will wear personal protective equipment (PPE) appropriate for the tasks and work environment.
What chemicals will you be using?
The calcination process adopted by Imerys limits the volume of acids used in the processes.














